分享:医用可降解锌合金的生物相容性评价研究进展
锌合金由于其适宜的腐蚀速率、良好的力学性能,成为继镁合金与铁合金后的一种新型的可降解生物医用金属材料。近年来,研究者对锌合金的设计、加工制备以及降解机理进行了大量研究,但对其体内外生物相容性的研究尚不充分,仅评估了体外细胞毒性、溶血、凝血,少数材料植入动物体内进行了组织相容性的表征。然而生物相容性涉及细胞、组织、血液、免疫等复杂的局部反应和全身反应,除了材料本身的物理化学性质,还受到材料与机体相互作用的影响。本文分析了可降解医用锌合金的化学成分和物相组成,阐明了对其进行生物学评价的方法,并结合锌合金生物相容性研究现状,阐述未来的研究方向。
关键词:
不锈钢、钛合金和钴铬合金等医用金属材料因其优越的力学性能、界面稳定性和良好的生物相容性,广泛应用于骨科及血管介入等植入性医疗器械[1,2]。然而,除了人工关节、脊柱内固定植入物等人体承重部位的器械需要提供永久支撑外,大多数时候人体对于植入物提供的支撑、固定等功能的需求只是暂时的。植入物长期存留体内还会带来不可预期的负面影响,例如骨折的病人再次骨折的风险增加1.4~2.5倍,其中重要的因素就是骨折内固定用的不锈钢板和螺钉与人体骨因弹性模量的巨大差异造成应力遮挡而引起骨质流失[3,4];约10%的患者支架术后存在内皮愈合不良或功能障碍导致的血管再狭窄和血栓,其原因即永久存在的支架平台对血管的持续刺激[5,6]。此外,永久支架相当于给血管套了“金属夹克(metal jacket)”,使血管舒张受限,特别是禁锢婴儿或儿童正在生长的血管[7,8]。锌合金是和镁合金、铁合金共同受到关注的一类可降解金属。由于其腐蚀速率介于镁合金与铁合金之间,具有较为理想的降解行为,成为近年来的研究热点[9,10]。Mg、Fe、Zn的性质见表1[11~15]。近年来,研究者对锌合金的设计、加工制备以及降解机理进行了大量的研究,但对其体内外生物相容性的研究尚不充分,仅评估了体外细胞毒性、溶血、凝血,只有少数材料植入动物体内进行了组织相容性的表征。然而锌合金器械在植入体内后,会引起一系列复杂的机体反应,同时机体生理活动中的力学运动、生物化学作用和生物电、磁场等作用会以各种形式影响其力学性能和降解行为,随着材料的降解和力学性能的丢失,可能对机体的组织或器官产生复杂而长期的影响。因此系统、全面的生物相容性评价关系着锌合金器械临床应用的安全性和有效性。
Table 1
| Metal | Standard electrode | Yield | Young's | Shear | Elastic | Hardness | Required | Develop |
|---|---|---|---|---|---|---|---|---|
| potential | strength | modulus | modulus | modulus | HV | element | -ability | |
| V | MPa | GPa | GPa | GPa | ||||
| Mg | -2.37 | 51-244 | 44-45.5 | 16-18 | 44-48 | 38 | Yes | No |
| Zn | -0.76 | 285-325 | 90-110 | 35-45 | 14-32 | 42 | Yes | Yes |
| Fe | -0.44 | 108-122 | 204-212 | 78-84 | 195-235 | 157 | Yes | Yes |
1 医用锌合金材料化学成分和物相组成分析
影响医疗器械生物相容性表现的最关键因素是用于制造器械的原材料的化学性质,在进行生物学试验之前鉴别材料化学成分并考虑其毒理学影响是非常重要的[16]。医用锌合金一般由高纯Zn粉(> 99.99%,质量分数)加一定比例的高纯无毒合金化元素熔炼成锌合金铸锭,经过塑性加工和热处理制成棒材、线材、板材等产品,再经过深加工变成多种植入器件[17~20]。在加工过程中,虽然有塑性加工冷却剂、轧制油等附着在表面,但经过清洗和表面打磨后,通常没有残留。
由于合金元素含量一般比较低,Zn(OH)2、ZnO、Zn3(PO4)2和ZnCO3是降解过程中的主要产物[21,22]。表2列出了锌合金主要降解产物的物理化学和毒理学性质。Zn(OH)2是锌合金降解的中间产物,常与ZnCO3共价结合ZnCO3·3Zn(OH)2。ZnO微溶于水,具有收敛性和抗菌能力,也是常用的营养增补剂。ZnCO3·3Zn(OH)2不溶于水,也无毒。相对于ZnO和Zn(OH)2,Zn3(PO4)2可溶于水,更容易被机体转运、吸收。一个锌支架大约50 mg,以全部转化为Zn2+计算,远低于导致全身毒性的安全阈值(ZnCl2的半数致死量约为500 g/人)。此外,Zn是人体必需的微量金属元素,组织分布广泛,锌合金植入物游离出来的锌离子可能还会参与维持机体生理功能和正常代谢[23]。人体内至少300种酶以Zn为辅助因子参与糖类、脂质、蛋白质和核酸代谢[24,25]。从DNA甲基化的表观遗传变化到发育,癌症进展/抑制,骨骼重塑/矿化和动脉粥样硬化,Zn在预防和治疗各种病理状况中起着关键作用[26,27]。Hennig等[28]的综述文章表明,Zn具有很强的抗动脉粥样硬化特性,作者认为,这种行为源于锌离子作为抗氧化剂和内皮细胞膜稳定剂的作用。另外,Zn也是骨骼形成和矿化必需的元素,能够刺激成骨细胞生长,同时抑制破骨细胞行使骨吸收的功能[29]。在大脑中,Zn可通过谷氨酸能神经元储存在特定的突触囊泡中,调节大脑的兴奋性,对大脑和中枢神经正常功能的发挥起着至关重要的作用[30,31]。Zn的组织分布和功能多样性为锌合金的临床应用提供了先决条件。锌合金的加工过程及其降解产物的性质表明了锌合金在人体中的应用具备基本的安全性,可以用来制做血管支架、腔道支架、软组织闭合和伤口修复、骨修复产品(夹、钉)等[32~35]。
表2 锌合金主要降解产物的性质
Table 2
| Degradation | Solubility, 20oC | Stability | Density | LD50, acute systemic | Equivalent to human | Toxicity |
|---|---|---|---|---|---|---|
| product | g·L-1 | g·cm-3 | toxicity, oral, rat | lethal dose | level | |
| mg·kg-1 | g | |||||
| ZnO | 0.0029, | Stable | 5.68 | > 2000 | 50 | Low toxicity |
| slightly soluble | ||||||
| ZnCO3·3Zn(OH)2 | Insoluble | Stable | - | > 10000 | 500 | Non-toxic |
| Zn3(PO4)2 | 2.7, | Stable | - | > 5000 | 500 | Non-toxic |
| slightly soluble | ||||||
| ZnCl2 | Soluble | Stable | 1.01 | > 5000 | 500 | Non-toxic |
2 生物相容性评价方法
根据ISO 10993-1:2018 的定义,生物相容性是指医疗器械或材料在一个特定应用中引起恰当宿主反应的能力[36]。图1[37,38]展示了材料植入后的机体反应及机体与材料(器械)的相互作用。当材料(器械)植入机体后,首先引起机体损伤修复,包括急性和慢性的炎症反应,通过适度的纤维包裹达到植入物与机体的稳态。整个过程中植入物会通过物理化学作用(溶出、降解等)、单纯的机械作用(运动、摩擦等)等影响机体应答,同时又会因机体的反应而影响材料本身[37,38]。植入物的生物相容性评价广义上讲是个动态的、长期的,贯穿研发、生产、临床应用全生命周期风险管理的过程[39]。
图1
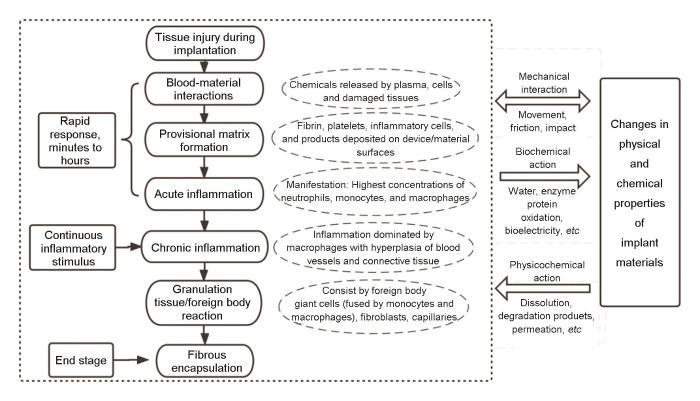
图1 材料植入后的机体反应及机体与材料的相互作用[37,38]
Fig.1 Body response to material implantation and body-material interactions[37,38]
2.1 生物相容性影响因素
影响医疗器械生物相容性的因素主要有:(1) 材料物理化学、形态学和表面特性表征;(2) 工艺过程中预期的添加剂,产生的工艺污染物和残留物;(3) 可沥滤物质;(4) 不同组件间的相互作用;(5) 终产品的性能与特点。器械中相关原材料、工艺污染物、可沥滤物的化学特性与生物相容性关系最为紧密,其与组织的接触性质、程度、频率和持续时间极大影响机体的反应[40]。有证据表明,一些金属元素,特别是Ni,可以刺激免疫系统,在包括过敏反应和炎症反应在内的不同病理的发生中发挥作用[41,42]。接受含Ni血管内支架的患者发生再狭窄一定程度上跟镍过敏有关[43,44]。除此之外,物理特性对器械的生物相容性也有较大影响,如多孔材料的孔径尺寸和孔隙率影响组织细胞的黏附、生长[45,46],磨损的颗粒尺寸和形状影响局部组织的炎症反应程度[47,48]。材料表面的拓扑结构如表面粗糙度、曲率及规则的几何图案影响蛋白质的黏附、细胞的增殖及形成血流的扰动[49,50]。血浆中的凝血因子蛋白在血液接触器件表面的快速吸附和激活是诱发血栓生成的主要途径[51,52]。值得注意的是,降解产物及周期影响植入物与人体的动态反应,一些可降解止血材料(如淀粉多糖、壳聚糖、再生氧化纤维素等)相较于非降解材料(如高密度聚乙烯)往往在植入试验中表现出程度更高、周期更长的炎症反应[53,54]。
2.2 生物学评价方法
目前我国对拟上市医疗器械的生物学评价采用GB/T 16886标准,该系列标准等同采用ISO 10993国际标准。根据GB/T 16886.1—2022 《医疗器械生物学评价 第一部分:风险管理过程中的评价与试验》对医疗器械按临床使用部位和接触时间分类,以选择合适的生物学试验项目。
表3列出了医用锌合金器械需考虑的必要生物学试验项目。对于医用锌合金制作的可降解的植入组织/骨的医疗器械,一般选取细胞毒性、皮肤刺激、迟发型超敏反应、急性全身毒性反应、(亚)慢性全身毒性反应、遗传毒性、植入后局部反应和降解。表4列出了需考虑的补充试验项目。例如,如果用于制造接触循环血液的支架,应增加血液学评价;如果用于制造宫内节育器、子宫托等与生殖系统有关的医疗器械,应增加生殖发育毒性的相关试验。理论上,还应考虑进行毒代动力学试验,但其必要性应结合器械/材料的预期用途,终产品化学组分,降解产物及可沥滤物(参照GB/T 16886.16—2021)。以支架为例,切割抛光好的锌支架重量大概50 mg,以全部是Zn元素计算,假设在1 a内完全降解,预期的每日剂量为140 μg,仅是成年男性推荐每日摄入量15 mg的1%。因此锌支架的植入并不会影响机体的血液系统、神经系统、消化系统、免疫系统等,如果锌支架在原位的降解过程没有引发强烈的炎症和过敏反应,即可认为无需进行毒代动力学的研究。同样的,只有当来自其他来源的数据表明有肿瘤诱导的倾向时,才应进行致癌性试验(参照GB/T16886.3—2019)。致癌性试验没有规定的标准和方法,可在动物试验中观察和记录原位组织和易感组织的肿瘤生成情况。
表3 锌合金植入器械必要的生物学试验项目
Table 3
| Test item | Current standard | Purpose and method | Judgement criterion | |
|---|---|---|---|---|
|
Cellular response |
Cell toxicity |
GB/ T16886.5—2017 |
The device/extract was cultured with cells, its potential cytotoxicity was evaluated by morphology and metabolic activity, such as MTT method | Cell viability > 70% was considered non-cytotoxic. For Zn-based materials, it is generally necessary to dilute the extract over a range of concentrations. Evaluate the result in vitro and in vivo comprehensively |
|
Tissue response |
Intradermal reaction |
GB/T16886.10—2017 | Intradermal injection of devices/materials extracts on the back skin of rabbits, to evaluate the non-specific percutaneous acute irritant effects of leachables | Erythema, edema, eschar, etc., were observed and scored according to the standard. The difference between the average scores of the test sample and the control should not be greater than 1.0 |
|
Implantation & degradation |
GB/ T16886.6—2015 |
Final devices/materials are implanted by surgical or interventional operation, and the target tissues are collected and observed at different time points to evaluate the local toxic effect of the sample on the living tissue and the degradation process (product) | Different degrees of tissue reactions (aseptic inflammation, fibrous cysts around the implant, etc.) will appear after implantion. With the influence of degradation, the reaction is higher and longer relatively. It's best to set a similar marketed product control | |
|
Immune response |
Delayed type hypersensitivity |
GB/T16886.10—2017 |
The immunity is usually induced by injecting the extract of the device/material plus protein to guinea pigs, and stimulated again after 2 weeks. Then the skin reaction is observed to evaluate the potential contact sensitization of the sample | No local skin erythema, edema and other inflammatory manifestations was considered to be no delayed type hypersensitivity reaction. Allergic reactions do not limit its use necessarily |
|
Systemic response |
Acute systemic toxicity |
GB/T16886.11—2011 |
Mouse is used routinely. Intravenous and intraperitoneal injection of the device/material extract is contacted with animals. The systemic response is observed to evaluate whether the sample releases toxic substances and produces acute systemic toxicity. The maximum exposure dose is 50 mL·kg-1 body weight |
Clinical performance (coat, skin, mucous membranes, respiration, muscles, behavior, etc.) should be observed and no indications. Gross pathological evaluation should be considered if clinically indicated |
|
(Sub)chronic systemic toxicity |
Rat is used routinely. The devices/materials or extracts are (repeatedly) contacted with animals by appropriate routes such as implantation, intravenous or intraperitoneal injection. The dose range is determined according to human safety limits. Clinical manifestations, body weight changes, hematological and clinical biochemical indicators, clinical pathological, gross pathological and histopathological analysis, etc., to evaluate whether the long-term exposure of sample to the human body will release toxic substances and produce (sub)chronic systemic toxicity |
Compared with the control group, no significant difference should be observed in each index |
||
|
Blood system |
Hemolysis |
GB/ T16886.4—2003 |
Direct contact of blood with the device/material or its extract, measuring the amount of hemoglobin released by erythrocytes to evaluate the degree of erythrocytelysis and hemoglobin release caused by the device/material |
Hemolysis rate should be < 5% |
|
Genetic system |
Genotoxicity |
GB/ T16886.3—2019 |
Mammalian or non-mammalian cells, bacteria, yeast or fungi are used to determine whether a device/material or extract causes genetic mutations, changes in chromosome structure and number, or other changes in DNA or genes. Bacterial gene mutation, chromosomal aberration and mouse lymphoma test are the most used in vitro tests |
There should be no significant difference compared to the negative control. If the in vitro test cannot be carried out or the results are confusing, further in vivo chromosome analysis and micronucleus test of mammalian bone marrow cells should be used |
表4 锌合金植入器械补充的生物学试验项目
Table 4
| Test item | Current standard | Purpose and method | Judgement criterion | |
|---|---|---|---|---|
|
Blood system |
Coagulation |
GB/ T16886.4—2003 |
The devices/materials are directly contacted with venous blood and poor platelet plasma (usually rabbits), respectively, and the clotting time is measured to evaluate whether the sample contains endogenous coagulation system activators |
Specify the acceptable criteria of the device/material on a verifiable basis (eg, compared to an approved device of the same type) |
|
Platelet adhesion |
The device/material is co-cultured with fresh sodium citrate anticoagulated whole blood (human, sheep or rabbit, etc.). The platelet adhesion on the surface of sample is observed to evaluate the effect of the sample on platelet performance | |||
|
Thrombosis |
The device/material is implanted into the vein. Thrombus formation on the surface of the sample and the intima surface of the blood vessel are observed and scored to evaluate the potential of forming thrombosis | |||
|
Complement system |
The device/material is contacted with human serum, and the concentration of C3a fragment formed during complement system activation is assessed by enzyme-linked immunosorbent assay to evaluate the effect of the sample on complement activation | |||
|
Reproductive system |
Reproductive toxicity |
GB/ T16886.3—2019 |
8-10 weeks before mating, male and female animals (mouse) are continuously exposed to device/material or extracts until 21 d after the birth of F1 generation. The sexual function, estrus cycle, mating behavior, conception, parturition, lactation, and weaning of animals as well as the growth, development, deformity, morbidity and mortality of offspring are observed and recorded, to evaluate the influence of the sample on the reproductive function and embryonic development |
There should be no significant difference compared to the negative control |
|
Metabolic system |
Toxicokinetics |
GB/T16886.16—2021 |
To study the quantitative changes in the process of absorption, distribution, metabolism and excretion of the test substance in the body, degradation products, leachables, and metabolites of device/material should be qualitatively detected and quantitatively analyzed. Rodent models (rats, mice) are generally used. Blood, urine, feces and bile are collected regularly after exposure, and the heart, liver, spleen, stomach, kidney, gastrointestinal tract, gonads, brain, body fat, skeletal muscle and other tissues are collected, respectively, to determine the distribution of the test substance. Bioavailability, toxicity-time curve, apparent volume of distribution, clearance rate, half-life, average residence time, maximum and maximum concentration (time) of the test substance were measured through the toxicokinetic model |
The mathematical model expression of metabolic process, combing with the physical and chemical shape, administration route, dose and method of the test substance is evaluated comprehensively |
医疗器械生物学试验的方法大多源自药物或化妆品的毒理学方法,样品制备方法也是针对不可降解材料,通过浸提的方法提取产品中的可沥滤物、加工助剂等可能影响产品生物相容性的物质。浸提过程受温度、时间、浸提介质(极性、非极性)、浸提比例以及材料的相平衡影响,因此GB/T 16886.12—2017对浸提液制备条件和方法进行了具体规定。由于锌合金在体内外降解行为的差异以及复杂的临床应用场景(血管支架、腔道支架、骨钉骨板、吻合钉、宫内节育器等),为避免受到不必要的限制或产生虚假的安全感,因此尚无针对该类产品的标准浸提液制备方法。当采用浸提液进行体内试验(如致敏、皮内反应、全身毒性、遗传毒性等)时,如浸提液中出现颗粒物无法直接用于试验,可考虑采用过滤、离心等方式去除,但应通过电感耦合等离子体发射光谱(ICP-OES)或电感耦合等离子体质谱(ICP-MS)等手段分析处理前后的元素组成和浓度,并进行方法学论证(参照GB/T 13748.15—2013)。如浸提原液具有细胞毒性,应分析其原因并对浸提原液进行梯度稀释(至无细胞毒性),再结合体内试验中植入物附近降解产物大量释放或积聚时对局部细胞活性及局部组织炎症反应的影响,对产品的生物安全性进行综合评价(参照GB/T 16886.15—2003)。
3 锌合金生物相容性研究现状
生物相容性评价最终以产品为导向,但目前锌合金植入器械只有用于颌面修复的骨板骨钉和心血管支架走到了临床试验阶段,未见其他产品报道进展。因此本文通过锌合金材料的生物相容性分析研究进展和未来的方向。
3.1 细胞毒性研究
细胞是发挥生物体功能的基本单位,细胞毒性是生物学试验中的首选项目,可为其他生物学危险源的识别和评估提供初步证据[55]。对于可降解医用金属器械/材料,细胞毒性主要受以下方面影响:金属表面性质如形貌、表面能、离子释放浓度、降解产物以及局部微环境(如pH值)变化[56~58]。其中,对细胞毒性影响最大的是离子浓度。根据对人体的作用分为有益金属离子(如钙、镁、铁、锌等离子)和有害金属离子(如汞、铬等离子)。即使是有益的金属离子,与人体的关系仍然存在剂量效应,当浓度太高时,也会因阻碍其他有益金属的结合而产生毒性。Ma等[59]研究了人主动脉内皮细胞(HAEC)在不同浓度(0~140 μmol/L) Zn2+环境中的短期细胞反应。低浓度Zn2+可使细胞活力、增殖、黏附、扩散和迁移能力增强,同时降低细胞黏附强度。然而,较高浓度Zn2+ (> 100 μmol/L)对HAEC行为表现出相反的影响(抑制细胞活力、增殖、迁移等)。Shearier等[60]研究显示,人体细胞对Zn2+的耐受性:HAEC (半数致死量LD50:265 μmol/L) > 平滑肌细胞(AoSMC) (LD50:70 μmol/L) > 真皮成纤维细胞(HDF) (LD50:50 μmol/L)。Kubasek等[61]研究表明,Zn2+对人骨肉瘤细胞(U-2 OS)和鼠成纤维细胞(L929)最大安全浓度分别为120和80 μmol/L。研究者们普遍认为不同的细胞品系针对锌合金材料会表现出不同的细胞反应,大概是由于不同类型的细胞尺寸、形态、细胞膜的转运过程、行使的功能以及细胞所处的微环境存在差异。这提示不同的可降解锌合金材料可能有其最佳应用场景,比如某一类材料更适合做骨科植入物、血管支架、腔道支架甚至宫内节育器。
在同一种应用场景下,有哪些措施可以改善锌合金器械/材料的细胞相容性呢? (1) 制备锌合金保护膜涂层。Shearier等[60]在金属Zn片上直接培养HAEC、AoSMC和HDF细胞,发现活细胞的数量几乎可以忽略不计。胶原蛋白和明胶表面修饰过的Zn片上,以上细胞均可附着和增殖。Jablonska等[62]在模拟体液(SBF)中预孵育Zn-1.5Mg合金,在表面形成保护性膜层,也可以减少离子释放量并增强初始细胞黏附。(2)添加适宜的合金化元素[63]。Shi等[64]制备了Zn-0.8Mn、Zn-0.8Mn-0.4Ag、Zn-0.8Mn-0.4Cu和Zn-0.8Mn-0.4Ca 4种合金,并在同一条件下进行细胞毒性试验。合金元素对细胞存活率的积极影响是Cu > Ca > Ag,在浸提液浓度为80%和60%时表现得最为显著。Li等[65]制备了纯Zn和Zn-4Ag的金属片,同样对其浸提液稀释3倍和L929细胞共培养2 d,纯Zn组细胞活力约106%,而Zn-4Ag组细胞活力约70%。这说明Ag对L929细胞的增殖有很强的负向作用。(3) 合金组织的影响。合金组织对体外细胞毒性最大的影响在于调控锌合金材料的降解性能,影响浸提液中Zn2+的含量。对于不可降解的合金,可以通过体外细胞直接接触法评估组织的影响。对于锌合金,因在细胞培养的过程中释放Zn2+则无法进行评估,只能通过原位植入的方式观察细胞的黏附、生长和迁移。
在研究阶段,锌合金材料表现出体外细胞毒性是否预示临床应用的安全性问题呢?不一定。人体血液中的Zn2+浓度约为120 μmol/L,心脏中高达400 μmol/L[66],远高于体外研究的细胞毒性浓度阈值。在体外细胞毒性试验中,锌合金接触的是开放的极性环境(细胞培养基),与长期植入体内的降解机制和降解产物有显著差异。另外,体外培养的单层细胞,失去了神经体液的调节和细胞间的相互影响,缺乏在体细胞动态平衡的相对稳定环境,对毒性极其敏感。表5分析了可降解医用金属器械/材料与传统不可降解医用金属器械/材料在细胞毒性试验方面的考虑。有研究[67]指出,当前ISO标准(例如ISO 10993-5或ISO 10993-12)不适用于可降解生物金属,需进行适当调整。例如对镁合金的细胞毒性评价,Wang等[68]系统地比较了体内外镁合金的降解速率、产物、pH值、渗透压的影响,按照ISO标准制备材料浸提液,再稀释10倍后与细胞共培养,以便对可降解镁合金进行较可靠的体外细胞毒性评估。通过体内外目标离子浓度的换算求得试验时“最接近临床应用状况”,可能同样适用于可降解锌合金。不同细胞的培养基成分不同,血清蛋白的种类和含量不同,会影响锌合金的降解和细胞对有毒物质的反应,因此建议进一步研究时采用统一的阳性对照做为标准参考,对锌合金临床应用会更具参考意义。
表5 可降解与传统金属植入器械/材料在细胞毒性试验中的异同
Table 5
| Item | Traditional metal implant devices/materials | Degradable metal implant devices/materials |
|---|---|---|
| Material type | Stainless steel, nickel-titanium alloy, cobalt-chromium alloy, titanium alloy, etc. | Zn alloy, Mg alloy, Fe alloy, etc. |
| Property | Inert | Bioactive |
|
Principle |
Support, occupy space, etc., by physical properties to achieve clinical therapeutic effects | Physical properties function in the early stage, then the materials degrade and the target lesion tissue remodels gradually |
|
Potential source of cytotoxicity |
Processing aids, leachables | Processing aids, leachables, degradation products (ions), pH, osmotic pressure, surface energy, etc. |
| Sterilization method | Not limited to ethylene oxide (EO), irradiation sterilization, etc. | Methods with minimal impact on material properties |
| Evaluation endpoint | Morphological evaluation, cell growth ability, and metabolic characteristics (microscopic observation + MTT method, etc.) | |
|
Contact way |
Extraction method, direct or indirect contact method can be selected according to the principle of "closest to the application situation". Generally, the extraction method is recommended | Extraction method, direct or indirect contact method can be selected according to the principle of "closest to the application situation" |
|
Extraction medium |
The ability to support cell growth and to extract both polar and non-polar substances | Cell culture medium with serum, generally. The ratio of serum can be adjusted according to the effect of serum on the material |
|
Extraction condition |
Generally (37 ± 1)oC, (24 ± 2) h |
The appropriate extraction time can be selected according to the implantation time, degradation rate and degradation products in vivo. Evaluation of multiple extraction times are also necessary to fully understand and assess biological risks |
| Extraction ratio | (Surface area or mass / volume) ± 10% | |
| Extraction | Dilution is not recommended generally | Multiple dilutions may be necessary |
| Extraction treatment | Adjustment is not recommended generally | Filtration, centrifugation, pH adjustment, etc., can be used, but treatment should be recorded and evaluated |
|
Cell line |
Suitable cell lines recognized by ISO experts, such as mouse fibroblast CCL1 (L929), mouse embryonic fibroblast CCL163 (Balb/3T3 clone A31), etc. L929 cells are generally used in China | Depending on the application site, sensitivity- or site-specific cells may be required to evaluate their cellular responses more objectively |
3.2 锌合金的血液相容性研究
根据ISO 10993-4的定义,血液相容性是血液对外源性物质或材料产生合乎要求的反应[26]。血液学机理和血液成分的功能比较复杂,可以明确的是血液中含有多种蛋白质,包括纤维蛋白原、血清白蛋白、免疫球蛋白、补体蛋白等。当血液相容性不良的材料与血液接触的数秒内,白蛋白、γ-球蛋白、纤维蛋白原等蛋白分子即在材料表面发生非特异性吸附,之后引发血小板及白细胞在材料表面的聚集、黏附和活化,继而凝血系统和纤溶系统激活,补体激活,以及诱发各种细胞因子参与的细胞免疫应答等[51,69,70]。这些事件高度相关,最终形成血栓和炎症反应。而血栓和炎症反应是关系骨植入和心血管植入材料体内应用成败的关键。
在一项以316L不锈钢、纯Zn和锌合金为对照的研究[83]中,系统评估了Zn-0.8Cu、Zn-0.8Mn和Zn-0.8Li 3种锌合金的血液相容性。研究显示,316L不锈钢、纯Zn、Zn-0.8Cu、Zn-0.8Mn和Zn-0.8Li的溶血率分别为0.38% ± 0.08%、1.04% ± 0.21%、0.47% ± 0.21%、0.57% ± 0.14%和0.52% ± 0.22%。在血小板黏附试验中,锌合金表面的血小板呈圆形,几乎没有伪足扩散。数量上,锌合金与316L不锈钢相比无统计学差异,但明显少于纯Zn组。并且,表面均未观察到明显的血小板活化、血细胞聚集、凝血或补体活化现象。此外,锌合金延长了血液的凝血酶原时间(PT)和部分凝血活酶时间(PTT),暗示了其潜在的抗凝功能。此外,其他研究[72~74]报道的锌合金材料的溶血性能均小于5%,符合ISO标准的要求。因此,锌合金材料的血液相容性良好,满足作为骨科和心血管植入材料的要求。值得一提的是,材料的加工工艺、表面特性等关系着医疗器械终产品的血液相容性的好坏。如锌合金材料制成了与循环血液直接接触的支架等,应基于终产品进行血液相容性的全面评价。
3.3 锌合金的体内试验
2013年,Bowen等[75]首次将纯Zn丝植入到Sprague-Dawley (SD)大鼠主动脉,6个月后Zn丝周围的组织反应良好,并没有明显的坏死。之后,研究人员又将纯Zn丝植入到大鼠腹主动脉6.5个月后进行观察,组织学检查表明植入的Zn丝与动脉组织具有良好的生物相容性,没有出现引发血管再狭窄的炎症反应、局部坏死和内膜增生,血管组织在支架降解部位再生良好。另外,Zn丝周围的细胞密度较低,明显缺乏平滑肌细胞,这表明Zn具有抗增殖作用,可有效防范支架植入后的再狭窄[76]。同样的方法植入纯Fe丝到大鼠的主动脉,铁丝附近无细胞和组织的长入,说明纯Zn比纯Fe在体内的生物相容性更好[77]。
为探索锌合金在血管支架方面的应用,Yang等[78]将纯Zn支架植入到兔子主动脉1 a时间,植入期间,兔子动脉内没有出现炎症、血小板聚集、血栓形成和明显的内膜增生,支架降解的过程中血管发生了良性重塑。此外,还发现了与血管重塑过程相匹配的Zn支架降解行为:前6个月内,纯Zn支架可以保持力学完整性,12个月后约40%的支架已经降解。纯Zn在体内的表现证明其做为可降解医用材料的优势,降解速率适宜,生物相容性良好。但是纯Zn的力学性能较差,需要加入合金化元素才能满足特定的应用需求。
Li、Mg、Al的加入(Zn-0.1Li、Zn-0.2Mg、Zn-0.5Mg、Zn-8Mg、Zn-1Al、Zn-3Al、Zn-5Al)改善了力学性能,却使得上述合金在体内(大鼠腹主动脉)的生物学表现更差[79~81]。植入11个月后,在Zn-Li合金组中仍观察到中等程度的慢性炎症反应,并伴非阻塞性的新生内膜增厚[79]。与纯Zn相比,Zn-xMg合金随着Mg含量的增加,生物相容性呈轻微恶化的趋势,炎症细胞浸润和新生内膜活化略有升高。植入6个月时,Zn-8Mg没有显示出明显的内膜增厚,但表现出中等程度的慢性炎症,以及管腔横截面积的减少。植入11个月时,炎症有一定程度消退,但出现内膜增厚,并伴有不连续的内皮细胞[80]。Zn-xAl表现略好,在植入3个月时,仍可观察到中性粒细胞和嗜酸性粒细胞浸润的急性局部炎症。植入6个月时,观察到植入物周围致密的纤维化沉积物,未检测到坏死组织,与周围动脉组织具有可接受的相容性[81]。
任何材料在植入体内后,均会引起机体一个急性的炎症反应,表现为材料与局部组织接触处的淋巴细胞和少量嗜酸性粒细胞浸润。对于生物相容性好的材料,1~2周后炎症逐渐消退,形成适度的纤维包裹,否则会引发更严重的急性炎症反应或长期的慢性炎症[82]。上述合金比纯Zn生物相容性差的原因大致在2个方面:(1) Li、Mg、Al元素的添加加速了锌合金的腐蚀,大量的腐蚀产物造成炎性细胞、巨噬细胞的聚集,无法在短时间清除和达成稳态;(2) 合金的显微组织、表面和晶间化合物可能对细胞产生一定的影响。例如,Mg2Zn11颗粒可能导致有害的巨噬细胞反应,破坏锌基金属传递的积极重构效应[80]。
在惰性气体保护下,以99.999%高纯Zn和99.995%高纯Cu为原料,经熔炼、轧制、挤出、拉拔、退火、激光雕刻、抛光、酸洗、环氧乙烷灭菌、解析等工艺流程制备的Zn-0.8Cu冠状动脉支架是目前唯一的一款已经中国国家药品监督管理局批准进入人体临床试验的锌合金血管支架。与兔、大鼠等小动物相比,猪的冠状动脉与人体冠状动脉在血管尺寸、解剖学特征、新生内膜生长方面最为相近,因此猪冠状动脉植入模型是评价支架的降解周期、力学性能、降解产物同组织的相容性及组织反应的重要手段[83]。Zn-0.8Cu支架植入猪的冠状动脉长达24个月,结果显示,支架植入后1个月内完成血管内皮化,在植入的24个月内提供了足够的结构支撑并显示出适当的降解速率。支架植入3个月后,支架杆周围聚集有细胞核深染的上皮细胞样巨噬细胞,植入9个月后巨噬细胞浸润减少,并转变为轻度炎症,支架杆降解部分被平滑肌细胞取代。降解产物在支架杆周围形成后,可以分解成小尺寸的产物(< 20 μm)并向血管外膜的方向扩散,而不是滞留在原位成为不易扩散和降解的大尺寸团聚物。腐蚀产物的向外迁移一般有2种可能性,一是通过细胞间途径中的体液,二是通过细胞路径中的细胞吞噬。研究观测到多核异物巨细胞吞噬Zn-0.8Cu支架降解产物碎片的直接证据。在支架植入后6、12和24个月时,检测Zn元素在植入段血管近端5 mm处血管、远端5 mm处血管和心尖部位组织的含量,结果显示,不论是Zn-0.8Cu支架组和不锈钢支架对照组之间,还是植入段血管的远端与近端之间,Zn元素的含量均无显著差异。光学相干断层成像(optical coherence tomography,OCT)观察到新生内膜光滑,没有发现血栓形成或严重的炎症反应[84]。Oliver等[85]观测到Cu在Zn-Ag基合金植入SD大鼠腹主动脉后炎症抑制(inflammation-resistance)的现象,通过细胞免疫荧光染色(immunofluorescence)等方法证实合金中的铜离子促进了植入物表面NO的产生,而NO能够抑制血小板黏附、平滑肌细胞增殖和白细胞黏附等。表6[75,76,78~81,84~86]总结了锌合金在血管中应用的动物试验。
表6 锌合金在血管中应用的动物试验总结[75,76,78~81,84~86]
Table 6
|
Material |
Shape |
Disinfection/sterilization |
Animal |
Implant site |
Implant period month |
Performance |
Ref. |
|---|---|---|---|---|---|---|---|
|
99.99% pure Zn |
Wire, Φ0.25 mm × 15 mm |
UV irradiation |
SD rats |
Abdominal aorta |
1.5, 3, 4.5, 6; 2.5, 4, 6.5 |
At 2.5 months of implantation, neoendothelialization was completed. The neointima contains a thin layer of SMCs and an area of low-density inflammatory cells adjacent to the zinc metal layer and within the corrosion layer, with no signs of necrosis. Despite rapid corrosion after 4 months implantation, the thickness of the neointimal layer did not increase over time. Migration and matrix formation of nucleated cells in the corroded area were observed. No inflammatory response, local necrosis, and progressive intimal hyperplasia were observed | |
|
99.99% pure Zn |
Long wire, Φ0.25 mm |
70% ethanol disinfection |
SD rats |
Abdominal aorta |
1~12, 14, 20 |
At 5, 6, and 8 months of implantation, there was higher cell density and chronic inflammation possibly related to stable corrosive activity. Chronic inflammation subsided between 10 and 20 months. No clear evidence of large-scale cytotoxicity was detected at any time point |
[86] |
|
99.995% pure Zn |
Stents, Φ3.0 mm × 10 mm, strut thickness: 165 μm |
- |
Japanese rabbits |
Abdominal aorta |
1, 3, 6, 12 |
No significant platelet adhesion or membranous thrombosis was observed after 3 d implantation. Neointimal coverage was observed at 1 month, indicating rapid endothelialization. No significant intimal hyperplasia or lumen loss was found at any time point, and no severe inflammation, platelet aggregation, or thrombosis was observed |
[78] |
|
Zn-0.1Li |
Wire, Φ0.25 mm × 10 mm |
- |
SD rats |
Abdominal aorta |
2, 4, 6.5, 9, 12 |
At 11 months postimplantation, moderate chronic inflammation with non-obstructive neointima was still observed in the Zn-Li alloy group. Biocompatibility is slightly worse than pure Zn |
[79] |
| Material | Shape | Disinfection/ | Animal | Implant site | Implant | Performance | Ref. |
| sterilization | period | ||||||
| month | |||||||
|
Zn, Zn-xMg (x = 0.2, 0.5, 8) |
Wire, 15 mm segment |
Disinfection |
SD rats |
Abdominal aorta |
1.5, 3, 4.5, 6, 11 |
Compared with pure Zn, the biocompatibility of Zn-xMg alloy showed a slight deterioration trend with the increase of Mg content. The inflammatory cell infiltration and neointima activation increased slightly. At 6 months, Zn-8Mg did not show significant intimal thickening, but exhibited moderate chronic inflammation and a reduction in the cross-sectional area of the lumen. At 11 months, inflammation had some resolution, but intimal thickening with discontinuous endothelial cells was appeared. It is speculated that Mg2Zn11 particles may induce deleterious macrophage responses thus disrupting the positive remodeling effect of Zn |
[80] |
|
Zn-xAl (x = 1, 3, 5) |
Strip, 12 mm × 300 μm × 300 μm |
70% ethanol disinfection |
SD rats |
Abdominal aorta |
1.5, 3, 4.5, 6 |
At 3 months of implantation, acute local inflammation with neutrophilic and eosinophilic infiltration was still observed. At 6 months, dense fibrotic deposits around the implant were observed, no necrotic tissue was detected. Zn-xAl had acceptable compatibility with surrounding arterial tissue |
[81] |
|
Zn-0.8Cu |
Stent, Φ3.0 mm × 20 mm, wall thickness: ~127 μm |
EO sterilization |
White pigs |
Coronary artery |
1, 3, 6, 9, 12, 18, 24 |
Vascular endothelialization was completed within 1 months after stent implantation. ZnCu stent provided adequate structural support and exhibited an appropriate rate of degradation within 24 months, with no accumulation of degradation products, thrombosis or inflammatory responses |
[84] |
|
Zn-4Ag, Zn-4Ag-0.6Mn, Zn-4Ag- 0.8Cu-0.6Mn-0.15Zr |
Wire, Φ0.25 mm × 15 mm |
Disinfection |
SD rats |
Abdominal aorta |
3, 6 |
At 6 months of implantation, a significant reduction of inflammatory activities was found in the quinary alloy relative to the other Zn-based materials. And inflammation, but not smooth muscle cell hyperplasia, is correlated with neointimal growth for the Zn-Ag-based alloys |
[85] |
尽管Zn-0.1Li在大鼠腹主动脉观察到长达11个月的慢性炎症反应和内膜增生,但是Zn-0.4Li金属片在大鼠股骨的表现非常好,纯Zn和Zn-0.4Li植入部位均未见骨溶解、变形、脱位或气影,与术后即刻相比,术后8周相邻皮质骨显示出更高的放射学密度,表明周围成骨。与纯Zn相比,Zn-0.4Li植入物周围观察到更多的胶原蛋白和新骨组织[87]。精加工成多孔支架的Zn-0.8Mn、Zn-0.8Sr合金也表现出令人满意的结果。Zn-0.8Mn支架植入大鼠股骨外侧髁处缺损,术后4周可见新骨形成,术后8和12周支架周围出现大量新骨组织,骨小梁也比纯Ti组更厚,在体内显示出良好的成骨性能和生物相容性,在器官中也没有锌离子积累[88]。Zn-0.8Sr支架同样表现出良好的骨缺损修复性能和骨生长趋势,无炎症反应[89]。Zn-0.5Mn植入大鼠胫骨4个月后,观察到健康的骨和血管,骨髓病理切片显示骨髓增生,不影响肝肾功能[90]。除此之外,Zn-0.4Fe、Zn-0.4Cu、Zn-2.0Ag、Zn-0.8Mg、Zn-0.8Ca、Zn-0.1Sr和Zn-0.1Mn在植入大鼠股骨后,也表现出良好的生物相容性,无骨溶解、畸形或脱位的迹象[91]。随着时间的推移,植入物周围的皮质骨变厚,放射学密度变高,表明周向成骨。以上结果支持了不同合金元素的锌合金在骨科的应用前景。
表7 锌合金在骨科及其他组织应用的动物试验总结[87~93]
Table 7
| Material | Shape | Disinfection/Sterilization | Animal |
Implant site |
Implant period |
Performance | Ref. |
|---|---|---|---|---|---|---|---|
|
Zn, Zn-0.4Li |
Rod, Φ1.6 mm × 15 mm |
- |
SD rats |
Femoral |
2 months |
Neither pure Zn nor Zn-0.4Li implanted sites showed osteolysis, deformation, dislocation, or air shadows. Compared to immediate postoperation, the adjacent cortical bone showed higher radiographic densities at 8 weeks postoperation, indicating peripheral osteogenesis. Compared with pure Zn, more collagen and new bone tissue were observed around the Zn-0.4Li implants |
[87] |
|
Zn-0.8Mn, Zn-0.8Sr |
Porous scaffold |
- |
Rats |
Femoral condyle |
4, 8, and 12 weeks |
New bone formation was observed at 4 weeks after Zn-0.8Mn implantation, and a large amount of new bone tissue was observed around the scaffold at 8 and 12 weeks postoperation. The trabecular bone was thicker than that of pure Ti group. Zn-0.8Mn scaffold showed good osteogenic performance and biocompatibility in vivo. Zn ions were not accumulated in the organs. Zn-0.8Sr scaffold also has good bone defect repair performance and growth tendency without inflammatory reaction |
[88, 89] |
|
Zn-0.5Mn |
Φ1.5 mm × 5 mm |
UV disinfection |
SD rats |
Tibia |
4 months |
After 4 months implantation, healthy bone and blood vessels were observed, bone marrow hyperplasia was showed by pathological sections, liver and kidney functions were not affected |
[90] |
|
Zn-0.4Fe, Zn-0.4Cu, Zn-2.0Ag, Zn-0.8Mg, Zn-0.8Ca, Zn-0.1Sr, Zn-0.4Li, Zn-0.1Mn |
Rod, Φ1.6 mm × 15 mm |
- |
SD rats |
Femoral |
2 months |
The cortical bone surrounding the implant thickened and radiographic dense increased, indicating circumferential osteogenesis. All implants were biocompatible with no evidence of osteolysis, deformity or dislocation. At 2 months implantation, new bone was formed and contacted the implants directly |
[91] |
|
Zn, Zn-2Fe |
Φ7 mm × 2 mm |
- |
Wistar rats | Subcutaneous tissue of back |
4, 8, 12, 18, 24 weeks |
No tissue inflammation or necrosis was observed |
[93] |
|
Zn-0.8Li-0.1Mn |
Gastroin-testinal staple |
Disinfection |
Mini fragrant pigs |
Gastrointestinal anastomosis |
3 d, 8 weeks, 12 weeks |
In the early stage after surgery, there were a small amount of inflammatory cells (mainly neutrophils and lymphocytes) and macrophages around the Zn-Li-Mn and Ti alloy nails. Inflammation cells around the Zn-Li-Mn alloy nails were slightly less than the Ti alloy group. At 12 weeks postoperation, new gastrointestinal tissues were found around the nails in both groups, the tissues healed well, and the number of inflammatory cells was significantly reduced |
[92] |
由Zn-0.8Li-0.1Mn研制的胃肠钉展示了锌基三元合金在胃肠道吻合中的应用[92]。术后12周,Zn-Li-Mn合金钉和钛合金钉周围均有少量炎症细胞,主要包括中性粒细胞和淋巴细胞,还有少量巨噬细胞,且Zn-Li-Mn合金钉周围的炎症细胞数量略少于钛合金钉。植入12周后,2组钉周围均有新的胃肠组织,组织愈合良好,炎症细胞数量明显减少。表7[87~93]展示了锌合金在骨科及其他组织中应用的动物试验总结。
4 总结与展望
适宜的腐蚀速率、良好的力学性能和生物活性,使锌合金成为被寄予厚望的可降解生物医用金属材料。近年来,研究者们对锌合金的成分设计、加工制备过程、内部组织结构、表面改性及降解机理等方面进行了大量系统研究,不断优化其力学性能、腐蚀速率及生物相容性,并积极拓展其临床应用范围。本文根据材料与机体相互作用的方式,总结了对锌合金器械进行生物相容性评价的原则和方法。但是材料植入后的机体反应及机体与材料(器械)的相互作用极其复杂,是一个联系的、动态的、长期的生理过程,从基础研究到产品定型和优化需要经历漫长的过程,对新型可降解植入器械的临床试验或者应用应该持乐观且谨慎的态度。展望未来,仍有几点生物相容性方面的考虑需要被重视。
(1) 尽管多数研究支持锌合金在动物体内的生物相容性,但其降解过程中腐蚀产物层中金属间颗粒的富集、吸收和转运及其对组织再生的影响仍然令人担忧。如何对锌合金降解产物进行荧光标记,追踪降解产物的吸收、转运过程及相应的生物学表现,将是一项重大课题。
(2) 终产品植入物在运动疲劳中产生的应力损伤是否引起局部腐蚀甚至点蚀,从而导致植入物提前失效引起机体更多的物理损伤也是生物相容性研究的一个方面。另外,锌合金植入物与其他金属植入物配合使用可能会产生电偶腐蚀,改变产品临床预期的降解速率,这种情况下的使用顺序、空间和时间距离的安全性也应该有所考虑。
(3) 体外的细胞毒性评价作为一套敏感的毒性筛选系统,其作用仍然不可小觑。如何考虑局部微环境中细胞类型、蛋白、溶解O2的影响,结合体内降解速率、降解产物积累和周围细胞的表现,建立适当的细胞毒性评价体系对于可降解锌合金的临床应用具有重要意义,也可以做为一项锌合金加工设计时的体外筛选系统。
(4) 锌合金降解过程中导致局部Zn2+浓度增加,Zn2+参与体内多种生理活动,应在时间效应和剂量效应下研究过量Zn2+触发的信号通路、蛋白合成、物质转运对机体的影响,是否会存在生理调控之外的信号通路激活,降解产物对周围细胞包括炎症细胞在分子水平的影响等。近年来分子生物学和生物信息学发展出的基因组学、蛋白质组学和代谢组学研究也可联合应用于识别降解产物潜在的基因和细胞毒性。
(5) 锌离子具有广谱抗菌的特点,如何在临床应用中减少植入材料表面细菌膜的形成从而降低植入物相关的感染也很有意义[94~98]。
来源--金属学报






 沪公网安备31011202020290号
沪公网安备31011202020290号
